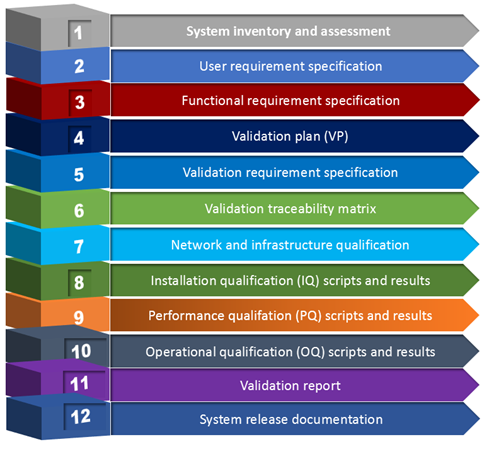
This training focuses on developing and implementing regulated computer systems with an appropriate level of documented evidence to satisfy FDA expectations. The core elements of computer validation program will be emphasized.
Topics to be discussed include:
Enhancing Customer Relationships via Technology Solutions.